Brand:-
Model:-
MOQ:-0 -
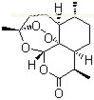
Artemisinin Anti Malaria Medication CAS 63968-64-9 Antimalarial Drugs
| Name | Artemisinin |
| Synonyms | (+)-Arteannuin; Qinghaosu; [3R-(3R,5aS,6S,8aS,9R,10R,12S,12aR**)]-Decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-one |
| Molecular Formula | C15H22O5 |
| Molecular Weight | 282.33 |
| CAS Registry Number | 63968-64-9 |
Description:
Artemisinin, also known as qinghaosu, and its semi-synthetic derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria. Treatments containing an artemisinin derivative (artemisinin-combination therapies, ACTs) are now standard treatment worldwide for P. falciparum malaria. Artemisinin is isolated from the plant Artemisia annua, sweet wormwood, an herb employed in Chinese traditional medicine. A precursor compound can be produced using genetically engineered yeast.
Application:
1. Applied in food field, in summer it will be serve as cool drinks which is good for prevention and treatment of heat stroke;
2 .Applied in health product field, artemisinin can be made into capsules or oral liquid to regulate the immune system;
3. Applied in pharmaceutical field, artemisinin has general effection for vivax malaria, plasmodium falciparum and chloroquine.
COMPANY INTRODUCTION:
Our company, as one of the most experienced exporter in China,specializes in API
and medicine intermediates. We export large quantity to USA,Europe and many other
countries all of the world, deal to high quality and reasonable price.
Patent Disclaimer:
1. Products protected by valid patents are not offered for sale in jurisdictions where the sale of such products constitutes a patent infringement. The current list only reflects the products and technologies that are available, under development: note that some products may be developed or produced for internal and experimental uses with no commercal aim.
2. Sales of products are limited to those allowed by Chapter VII PLPRC 63, the above includes Research and development quantities.
3. R&D use in accordance with (1) 35 USC 271(e)+A13(1) in the U.S.; (2) Section 69.1 of Japanese Patent Law in Japan; (3) Section 11, No. 2 of the German Patent Act of 1981 in Germany; (iv) Section 60, Paragraph 5b of the U.K. Patents Act of 1977 in the U.K.; (4) Sections 55.2(1) and 55.2(6) and other common law exemptions of Canadian patent law; (5) Section 68B of the Patents Act of 1953 in New Zealand together with the amendment of same by the Statutes Amendment Bill of 2002; (6) such related legislation and/or case law as may be or become applicable in the aforementioned countries; and (7) such similar laws and rules as may apply in various other countries.