MOQ:1
Specifications
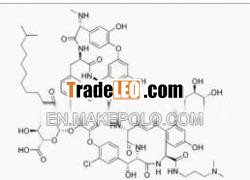
Dalbavancin1.CAS:171500-79-1
2.Usage:Antibiotic
3.Molecular Formula:C88H100Cl2N10O28
171500-79-1 lipoglycopeptide antibiotic ProductDalbavancin
Specification:
Dalbavancin (INN,trade name Zeven) is a novelsecond-generation lipoglycopeptide antibiotic. It belongs to the same classas vancomycin, the most widely-usedand one of the few treatments available to patients infectedwithmethicillin-resistant Staphylococcusaureus (MRSA).
Dalbavancin(BI397) is a novel semisynthetic lipoglycopeptide that was designedto improve upon the naturalglycopeptides currentlyavailable, vancomycin and teicoplanin.
It possessesin vitro activity against a variety of Gram-positive pathogens including MRSA and MRSE. Itis a once-weekly, two-dose antibiotic that Pfizer acquired when it bought Vicuron Pharmaceuticals in2005.
Dalbavancin has undergone a phase III clinical trial for adultswith complicated skin infections, but in Dec 2007the FDA saidmore data was needed before approval.onSeptember 9, 2008, Pfizer announced that it will withdraw allmarketing applications in order to conduct another Phase 3 clinicaltrial. DurataTherapeutics acquiredthe rights to dalbavancin in December 2009 and has initiated twonew Phase III clinical trials for treatmentof acute bacterial skin and skinstructure infections.Preliminaryresults in Dec 2012 looked good.
Storage condition: keepin closed containers at room temperature, avoid direct heat andlight.